 Biology 381, Pollution Biology
Biology 381, Pollution Biology Biology 381, Pollution Biology
Biology 381, Pollution BiologyDepartment of Biological Sciences,
Faculty of Science,
University of Alberta,
Edmonton, Alberta.
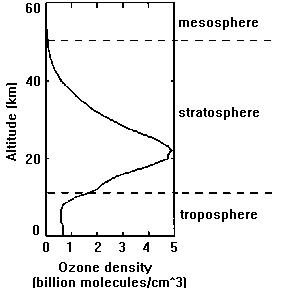
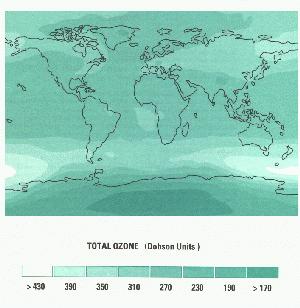

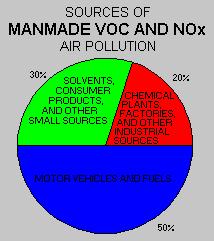
NO2 + hv _______________ NO + O.
O. + O2 _______________ O3
O3 + NO _______________ NO2 + O2
___________________________________
No net change...
NO2 + hv _______________
NO + O.
O. + O2 _______________ O3
NO + HxCx _______________ PANs
_______________________________________________
NO2 + O2 + HxCx + hv _______________
O3 + PANs
Insert figure
Insert figure.
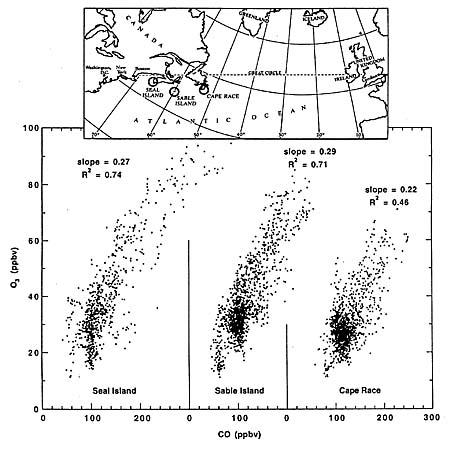
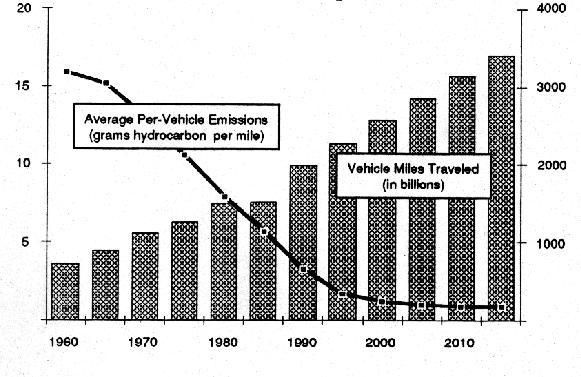
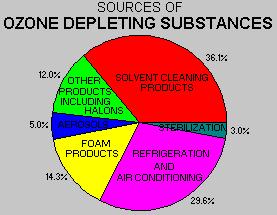
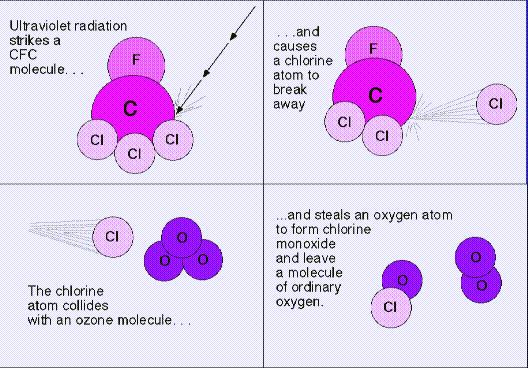
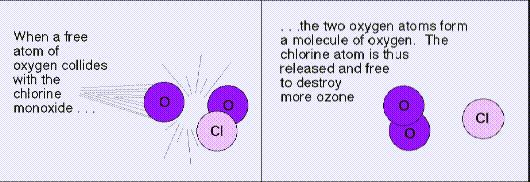
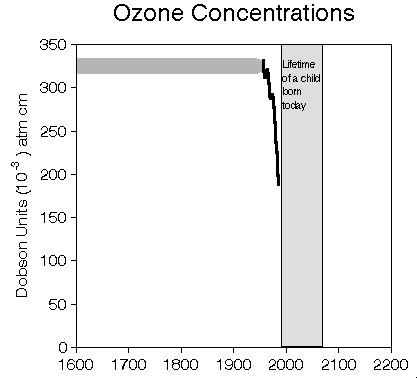
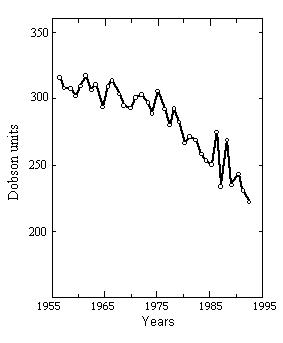
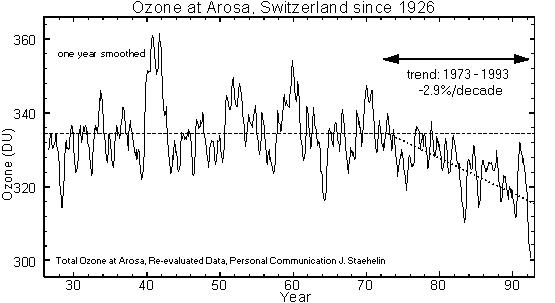

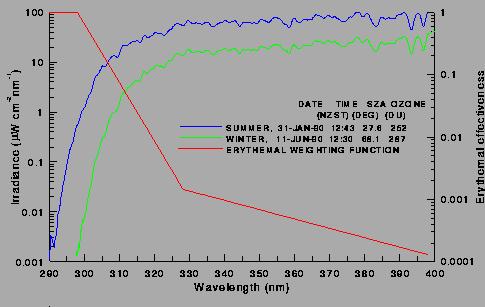
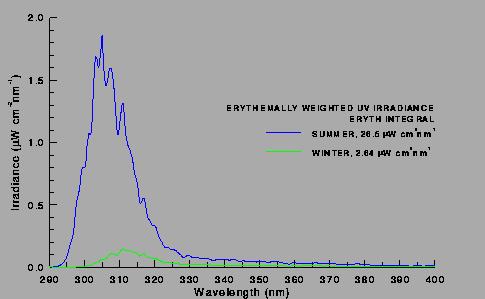
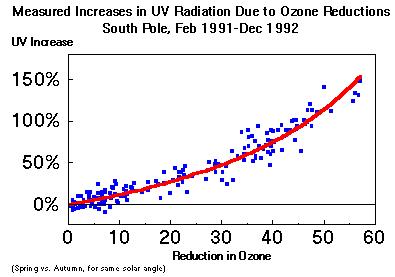
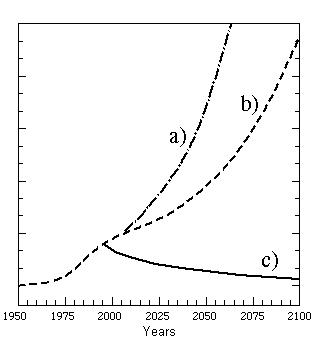
a) without restrictions on release of ozone-depleting chemicals,
b) according to the original Montreal Protocol of 1987, and
c) according to the revised Montreal Protocol
The U.S. Environmental Protection Agency's web page on the science of ozone depletion. - A wealth of useful data and information.
The U.S. Environmental Protection Agency's ozone depletion glossary. - Do you need any definitions?
WMO/UNEP Scientific Assessment of Ozone Depletion: 1994. - An executive summary to the bible of ozone depletion.
NASA's Facts about ozone- A good review. You'll recognize some of the figures from this site.
Global Resources frequently asked questions about ozone depletion.- A few tasty morsels.
Global 2000 view of a "critical issue." - A few more tasty morsels.
Last updated, January 29, 1997.