Biology 381
Pollution Biology
Department of Biological Sciences
University of Alberta
Edmonton, Alberta
 |
Biology 381 |
| 4. An inventory of Pollutants. |
4.1 Required Reading and other announcements.
No required reading.
4.2 What is pollution?
Pollution is "the unfavourable alteration of our surroundings, wholly or largely as a by-product of man's action. Who determines what is favourable and what it unfavourable?




Current pollution inventories are based upon a number of artificial criteria.
The source of pollution.
The resource that becomes polluted.
Figure IV-1, Nebel (1987).
The impact of the pollutant.
The chemistry of the pollutant.
Some of these inventories might be considered simple others might be considered intuitive, comprehensive, or definitive. None can claim all of these characteristics.
Today's literature contains elements of each and is packed with jargon reflecting preferences of individual authors.
4.4 Atmospheric pollution has changed the way we speak.
Figure 13-8, Nebel (1987).
When organic material is burned, the primary waste products are carbon dioxide (CO2) and water vapour (H20). However, burning is seldom complete. Other primary products of combustion may include:Particulates
Particulates are solid particles suspended in air (diameter < 0.00l mm). Particulates are normally composed primarily of carbon, but also include metals. Smaller particulates, which can be inhaled deeply, can cause lung damage and bronchitis with elevated concentrations over an extended period of time.
![]() Hydrocarbons
Hydrocarbons
Many hydrocarbons are unburned or partially oxidized organic fragments (primary products of combustion), others are synthesized for end use purposes.
Pesticides, chlorinated solvents (i.e. carbon tetrachloride, tetrachloroethylene, trichloroethane, methylene chloride), polychlorinated biphenyls (PCBs), aromatic compounds (benzene, toluene, xylene) polycyclic aromatic hydrocarbons (PAHs), and dioxins all pose a risk to human health. Volatile organic compounds (VOCs) have a variety of direct harmful effects, depending on the chemicals involved, concentration and length of time exposed. Reactions involving VOCs in the lower atmosphere lead to the formation of tropospheric ozone, which has hazardous effects on human health. VOCs (CFCs, HCFCs, CH4) and some of the chlorinated solvents (methylene chloride, trichloroethane) can also act as potent heat trapping gases.
![]() Carbon monoxide
Carbon monoxide
Carbon monoxide interferes with the blood's ability to transport oxygen to cells and tissues.
At high temperatures of combustion, nitrogen gas (N2) is oxidized to form nitric oxide (NO), nitrogen dioxide (NO2), and nitrogen tetroxide (N2O4). Nitric oxide (NO) formed inside automobile engines and boilers of coal-burning power plants slowly reacts with oxygen to form nitrogen dioxide (NO2).
Nitrogen oxides, at elevated concentrations, can cause irritation in the respiratory system, leading to bronchitis and other acute respiratory tract infections.
NO2 is responsible for the brownish haze over many cities. It can also react with water vapour to form nitric acid and plays a role in formation of ozone.
Sulphur impurities in fuels may be oxidized at high temperatures to form SO2. SO2 is a powerful irritant, reflecting the rapidity with which it forms sulfurous acid on contact with moist membranes. SO2 is a contributing component of acid rain. A variety of secondary products of combustion may form by reactions after combustion has occurred.
Atmospheric acids.
Both nitrogen oxides and sulphur dioxides react with water vapour in the atmosphere to form nitric acid and sulphuric acid respectively.
Photochemical oxidants
Photochemical oxidants are strong oxidizing agents that arise from secondary reactions in the atmosphere.
Ozone and peroxyacetyl nitrates are formed as a result of chemical reactions between nitrogen oxides and volatile hydrocarbons.
Figure 13-6, Nebel (1987)
Photochemical oxidants are important components of photochemical smog.
Photochemical smog is a relatively new form of air pollution.
The term "smog" was first coined to describe the smoke and fog over London.
Today, the term "photochemical smog" is used to describe pollution episodes which are characterized by a broad range of constituents, including both primary and secondary products of combustion.
Photochemical smog develops when primary pollutants (oxides of nitrogen and volatile organic compounds) interact under the influence of sunlight to produce a mixture of secondary pollutants.
Certain conditions are required for the formation of photochemical smog.
High concentration of nitrogen oxides (NOx) and volatile organic hydrocarbons (VOCs)
Sunlight and temperatures above 18C
Meteorological factors
Topography can also play a role
Nitrogen dioxide (NO2) is responsible for the brownish haze that hangs over many cities during the afternoon of sunny days.
Ozone, one of the major components of photochemical smog is a highly destructive and dangerous pollutant,which can cause severe damage to biological systems.
Smog events are not always restricted to large cities.
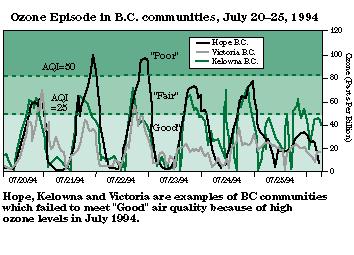
http://www.env.gov.bc.ca/~cvf/fig6.gif
The nuclear winter is a hypothetical environmental issue.
The Stokes Test, conducted on August 7, 1957 was a 19 kiloton device exploded from a balloon. http://www.nv.doe.gov/news&pubs/photos&films/atm.htm
The nuclear winter predicted to follow a nuclear exchange would arise from massive ejection of particulates into the atmosphere.
Figure 14-29, Nebel (1987)
Synthetic organic chemicals used as pesticides and herbicides are relatively new to society (100 years).
All pesticides are highly toxic, acting as a nerve poisons and causing birth defects and cancer.
Chlorinated hydrocarbons, including DDT, Chlordane, and Mirex have a long half life in the environment, (DDT 3 - 20 years). Their structure makes them extremely resistant to break down.
This and several other properties accounts for their tendency to biomagnify up the food chain.
Organic phosphates, including Malathion and Parathion, break down more readily than the chlorinated hydrocarbons, but they are still passed up the food chain.
The Carbamates are the most widely used pesticides today (Severn, Carbaryl). These pesticides break down rapidly (days to weeks) and rarely passed up the food chain.
4.6 Nature turned nasty.
Sediments and water pollution.EutrophicationSoil erosion occurs even in undisturbed, natural ecosystems, but at a much lower level than in disturbed sites.
Sediment movement has compound effects on aquatic ecosystems.
Figure 9-1, Nebel (1987)
Eutrophication of aquatic ecosystems results from the addition of nutrient-rich material to water bodies.
Figure 11-3, Nebel (1987).
Increased supplies of nutrients stimulates growth of algae.
Their eventual death creates an oversupply of detritus.
Increased rates of decomposition deplete dissolved oxygen
4.7 Additional world wide web information.
Canada's National Pollution Release inventory. A Canadian database providing information about release of pollutants into our air, water and land (1993-1997).
Canada's Toxic Substances Management Policy. This policy provides decision makers with direction and sets out a science-based management framework to ensure that federal programs are consistent with its objectives.
Agency for Toxic Substances and Disease Registry (ATSDR). Every two years, the U.S. EPA compiles a list of substances that pose the most significant potential threat to human health. Links to information about the top 20 on this list are provided.
Agency for Toxic Substances and Disease Registry (ATSDR). The complete list (n=275) prioritized by risk. Links to web based information are provided separately.
A summary of major sources of pollution. A wonderful review of the contributions of power plants, industry and waste disposal, transportation, domestic sources, and agriculture to air pollution in Europe.